CDK19 in Mitochondrial Dynamics: Unraveling Its Role in Neurological Disorders
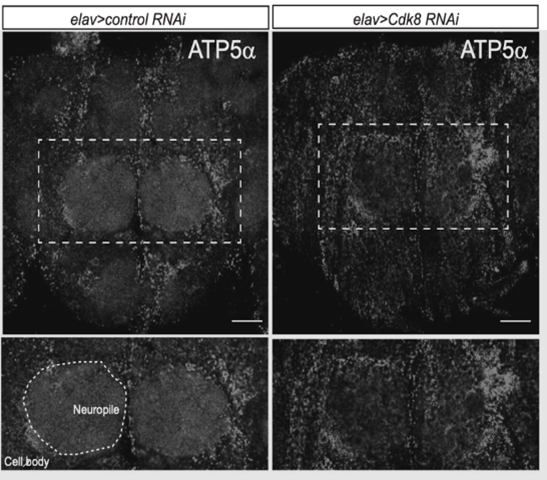
Research focuses on the role of CDK19/Cdk8 in mitochondrial fission, fusion, and its impact on neurological diseases. Recent findings show CDK19 localizing in the cytoplasm and mitochondria of neurons, suggesting its role in maintaining mitochondrial integrity. The study explores CDK19’s influence on mitochondrial dynamics and its relevance to both rare CDK19-related neurological disorders and Parkinson’s disease, where mitochondrial dysfunction plays a key role. The research aims to uncover how CDK19 contributes to these conditions, offering potential targets for treatment.
Reference:
– Chung HL, et al. Am J Hum Genet 2020
– Chung HL, et al. Neuron 2020
– Chung HL*,#, et al. Cell Metabolism 2024
Discovery of new rare human disease-causing genes using novel genetic approaches in Drosophila
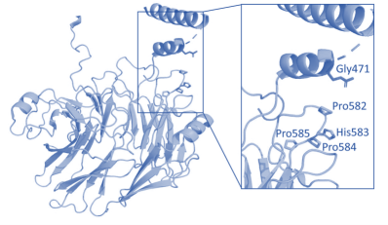
Research focuses on discovering new disease-causing genes using Drosophila models, leading to key findings such as EMC1, DNMBP, and others. Through international collaborations, the work has expanded globally, linking human genetic data with fly models to provide insights for interpreting rare genetic variants. Research on sphingolipids in the nervous system offers novel therapeutic opportunities for rare neurological diseases, including Mitchell Syndrome. Advanced techniques like CRISPR-Cas9 and collaborations with platforms like GeneMatcher are used to accelerate the translation of research outcomes into clinical practice, advancing the understanding of neurodegenerative diseases.
Reference:
– Chung HL, et al. Human Molecular Genetics 2022
– Ansar M*, Chung HL* et al. Am J Hum Genet 2018
– Ansar M*, Chung HL* et al. Am J Hum Genet 2019

Lipid metabolism in rare and common neurologic disorders
Research focuses on using Drosophila melanogaster to uncover rare genetic disorders tied to lipid metabolism. The team discovered Mitchell Syndrome, revealing a new role for peroxisomes in glial cells and their impact on neuronal health. Key findings show how elevated VLCFAs in myelin are converted into S1P, driving neuroinflammation. Targeting the S1P pathway in Drosophila and mouse models has led to FDA approval for compassionate use of N-acetyl cysteine amide in Mitchell Syndrome. This groundbreaking research, featured in an NBC documentary, supported by the Mitchell and Friends Foundation, continues to explore advanced treatments like ASOs for a comprehensive cure.
Reference:
– Chung HL, et al. Cell Metabolism 2024
– Chung HL, et al. Neuron 2022

Functional studies of Hippo signaling components to provide mechanistic insights into basic biology and cancer
Research focuses on the Hippo signaling pathway, a critical tumor-suppressor mechanism that controls organ growth and regeneration. A key discovery was identifying a novel component of the pathway, offering new insights into how cells regulate growth and stop proliferation. This breakthrough has advanced the understanding of conditions like cancer and tissue regeneration disorders, opening doors for targeted therapeutic interventions.
Reference:
– Chung HL, et al. Developmental Cell 2016
– Chung HL, et al. Cell Cycle 2016
